Background: Mixed phenotype acute leukemia (MPAL) is a rare and heterogeneous type of acute leukemia that displays an ambiguous pattern of antigen expression to a degree that assigning a single lineage of origin is not possible due to which, understanding of the pathophysiology of the disease, diagnosis and treatment are limited. The lack of well-established optimal treatment for MPAL has paved path for requirement of a more effective and less toxic approach. Blinatumomab, a bispecific CD19-directed CD3 T-cell engager (BiTE) antibody and venetoclax, a potent, highly selective B-cell leukemia/lymphoma-2 (BCL2) inhibitor was approved for the treatment relapsed/refractory (r/r) B-acute lymphoblastic leukemia (B-ALL) and chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), respectively. Here, we present 6 patients with CD19-positive MPAL that are successfully treated with blinatumomab in combination with venetoclax and/or azacytidine in front line setting.
Methods: All 6 patients met the criteria for MPAL as defined by European Group for Immunological Characterization of Acute Leukemias (EGIL) or World Health Organization (WHO 2022). Flow cytometry and polymerase chain reaction (PCR) was used to assess MRD and BCR::ABL1 or KMT2A gene, respectively. As pre-phase treatment, patients received glucocorticoid for 7 days. Blinatumomab at a dose of 9 mg/day for 1 week followed by 28 mg/day for 3 weeks combined with venetoclax at a dose of 100 mg/day for 14 to 21 days and/or azacytidine at a dose of 75 mg/m 2/day was given as induction therapy subcutaneously for 7 days. The patient with BCR::ABL1 received blinatumomab in combination with olverembatinib. Intrathecal (IT) chemotherapy with cytarabine and methotrexate was administered to central nervous system (CNS) as prophylaxis treatment.
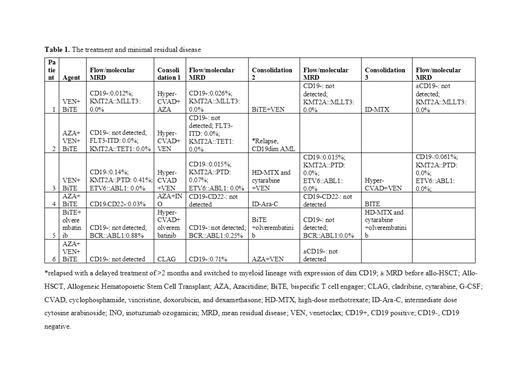
Results: Of the six patients included five were male and only one was female; five were diagnosed with B-cell/myeloid MPAL, while one with B-cell/T-cell MPAL. Abnormalities in KMT2A gene was observed in three patients while one patient had abnormality in BCR::ABL1 gene, two patients were negative for both gene abnormalities. After one cycle of blinatumomab combination treatment, all the six patients achieved complete response (CR) with full hematologic recovery. In 5 patients, MRD was <0.1% of these 3 had MRD-negative remission (<0.01%) and two patients had molecular remission. Patients were switched to chemotherapy post induction therapy, after one cycle of post-reinduction, the patients who still had the CD19-positive flow MRD or persistent molecular MRD received sequencing chemotherapy and blinatumomab. The treatment and MRD was presented in table 1. The median follow-up time was 10 months. Except 2 patients, all other patients achieved continuous MRD and molecular remission, of which two patients underwent an allogeneic hematopoietic stem cell transplant (allo-HSCT). The most frequent grade 3 or 4 adverse events (AEs) observed were anemia (6 patients), neutropenia (6 patients) and thrombocytopenia (4 patients). No AEs led to treatment interruptions and no serious AEs were reported.
Conclusion: The results provide preliminary evidence of effective and safe treatment with blinatumomab and venetoclax combination as first-line therapy in MPAL resulting in the achievement of normal bone marrow function with less toxicity than using conventional chemotherapy.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Blinatumomab, a bispecific CD19-directed CD3 T-cell engager (BiTE) antibody and venetoclax, a potent, highly selective B-cell leukemia/lymphoma-2 (BCL2) inhibitor was approved for the treatment relapsed/refractory (r/r) B-acute lymphoblastic leukemia (B-ALL) and chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), respectively. Here, we present 6 patients with CD19-positive MPAL that are successfully treated with blinatumomab in combination with venetoclax and/or azacytidine in front line setting.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal